|
|
MDS Project Case Studies
(Highlights from twenty-five years of success!)
Uresil, LLC: TRU-SET Suture Retention Catheter
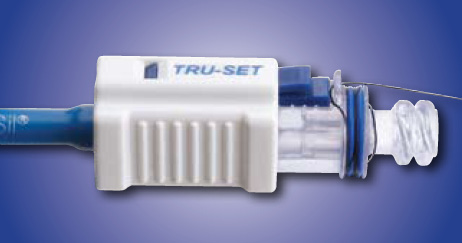
Case Study 7: Uresil TRU-SET™ Suture Retention Catheter Development
(see lock images below)
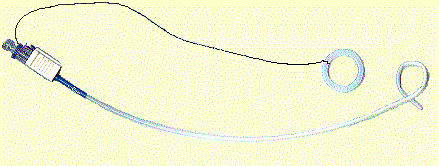
Suture Retention Catheters are used as body fluid draining devices. The catheter is inserted through body tissue in a
straight configuration. During insertion a "needle" resides in the bore of the catheter and protrudes from the catheter tip. The
sharp "needle" opens a passageway through the tissue for the catheter tubing. After insertion, the catheter tube is retained in the body by
curling the indwelling end of the catheter into a "pig tail" configuration. Using a grasping ring, a small string (suture) is pulled to curl the
catheter tip back on itself to form the "pig tail" shape. The suture is approximately two human hairs in diameter and made from a very slick material.
Once the "pigtail" is formed, the suture needs to be locked to prevent the catheter from working its way out of the body. At time of catheter removal,
the lock needs to readily open so that the "pigtail" unwinds to allow catheter extraction. The small diameter and
slick nature of the suture presented a challenge to developing a lock mechanism. Suture locking devices are located on the non-indwelling
end of the Suture Retention Catheter. These locking devices must be very small in size. The locks must be highly reliable.
The design requirements pushed the strength limits of plastic materials.
Medical Development Services, Inc. was contracted by Uresil, LLC to design a new locking device for their
existing Suture Retention Catheter Product line. The project commenced in September 2007. Uresil provided MDS with
design requirements. MDS initially worked to create a locking concept which could meet the design requirements. Lock concept feasibility
was demonstrated using hand made models that were constructed at MDS. MDS personnel worked seamlessly via the Internet and
through teleconferences with Uresil personnel and with material suppliers. Once a working concept was agreed upon, MDS produced
the SolidWorks CAD Models, identified component materials, and computer stress tested the locking device parts. New materials had to be
located and new processes had to be created to satisfy some of the part design. After creating the SolidWorks CAD models, the plastic
parts were constructed in rapid prototyping plastic models. MDS made a non-plastic part using processes devised at MDS. The
lock assembly had both grip and seal internal parts. The TRU-SET™ lock was designed for easy rapid production line assembly.
Testing performed on the Stereolithography prototype lock assemblies produced high confidence that the lock design would function
per the design requirements.
MDS personnel worked with Uresil part suppliers to assure that the part suppliers could fabricate the components in their high volume injection molding
processes. Uresil manufacturing engineers and Uresil part suppliers reviewed each design iteration and their recommendations were incorporated
by MDS in the final part design and assembly. Although many of the Virtual team members had never met in person, very few issues were encountered
during the project duration. Each organization and individual completed their tasks in reasonable time and communication was fast, efficient, and seamless.
MDS project work ended in September 2008 with transfer of final part and assembly SolidWorks models to Uresil and
part suppliers. During production tool construction, MDS supported Uresil in filing a new Patent application on the TRU-SET™ locking device design.
Uresil performed all FDA clearance testing and documentation requirements. The TRU-SET™ Suture Retention Catheter product introduction occurred on March 8, 2009 at the
Interventional Radiology Convention in San Diego, CA. MDS personnel attended the product introduction.
During the year long product development effort, MDS as a company did zero air travel for the project and used only eight gallons of gasoline in
local travel to acquire parts and materials. Uresil received the financial benefits of no travel and no facility overhead cost or travel time loss for
the MDS portion of the project. By utilizing MDS as an extended resource, Uresil's personnel focus was on daily operations.
The TRU-SET™ project was a successful endeavor between organization, using high tech processes and telecommunication. This type of collaboration has a definite affect on Green House Gas reduction. More business processes could be adapted to meet modern needs.
|
|